Enumeral Biomedical Holdings, Inc. (ENUM) is a development stage biotech company with a focus on immunotherapy for cancer and inflammatory disease. The company has developed a proprietary platform technology with the ability to screen antibodies at a single cell level, which holds multiple advantages over existing technologies. Based on this platform technology, Enumeral is currently focused on therapeutic discovery and immune profiling with the goal to build a robust pipeline rapidly and in a cost-effective way.
Currently, the discovery is focused on antibodies targeting immune checkpoints of PD-1, OX40, and Lag-3. The company plans to file an IND and initiate a Phase 1 clinical trial for PD-1 in 2016 in collaboration with a co-development partner. Meanwhile, we see platform validation on multiple fronts that we believe will lead to valuation expansion and upward movement in the shares over the next several quarters.
Phase 2 SBIR Grant With NCI
In September 2014, Enumeral was awarded a Phase 2 Small Business Innovation Research (SBIR) contract from the National Cancer Institute (NCI) for $999,967 over two years to develop an advanced, automated prototype system for human tissue immuno-oncology profiling. This program complements Enumeral’s other internal research and development efforts and enables it to continue its programs in colorectal cancer immune profiling that were initiated under the Phase 1 contract. Since the signing of the contract, Enumeral has been putting the pieces of the research effort into place. For example:
– In January 2015, Enumeral announced that the company signed an agreement with Memorial Sloan Kettering (MSK) Cancer Center as part of the Phase 2 SBIR contract with the NCI. Under the contract, Enumeral is developing an advanced, automated prototype system for human tissue immuno-oncology profiling, which will be deployed at MSK in the laboratory of Jedd D. Wolchok, M.D., Ph.D., Chief of Melanoma and Immunotherapeutics Service, Lloyd J. Old/Ludwig Chair in Clinical Investigation Department of Medicine and Ludwig Center, at Memorial Sloan Kettering Cancer Center. Dr. Wolchok played a critical role in the clinical development of ipilimumab (sold by Bristol-Myers as Yervoy®) and nivolumab (sold by Bristol-Myers as Opdivo®), immunotherapies that represent a transformational advancement in the treatment of cancer.
– In March 2015, the company announced an agreement with the Ragon Institute of Massachusetts General Hospital (MGH), Massachusetts Institute of Technology (MIT) and Harvard University as part of the SBIR contract with the NCI. Enumeral is developing an advanced, automated prototype system for human tissue immuno-oncology profiling, which will be deployed in the laboratory of Douglas S. Kwon, M.D., Ph.D., Assistant Professor of Medicine at the Ragon Institute. The Ragon Institute’s research focus is on the immunology of mucosal surfaces and tissues, which are home to between 60-90% of the body’s lymphocytes, the body’s primary disease-fighting white blood cells. Studying these immunological compartments in human patients relies on “small volume” clinical specimens such as core biopsy, which often contain limited numbers of cells and can be challenging to analyze with conventional technologies. Through work done by Ragon and Enumeral’s “Immune system on a chip” platform, the collaboration hopes to improve the understanding of the fundamental biology of the human mucosal immune microenvironment, which in turn could impact the diagnosis and treatment of infectious diseases, inflammatory diseases, and cancers.
Collaboration With Merck
On December 18, 2014, Enumeral Bio announced that it has signed an oncology-focused collaborative study agreement with Merck. The collaboration includes use Enumeral’s human approach to interrogating the tumor microenvironment in colorectal cancer tissues obtained directly from patients in order to identify functional cellular responses to immuno-oncology therapies being developed by Merck. Enumeral’s human-driven immune profiling platform may increase the probability of finding rare immune cells associated with a disease or drug response through improved efficiency and sensitivity. Studying rare cells obtained directly from human patients for their functional responses can potentially lead to identification of those patients responsive to a therapy, while elucidating the underlying cellular basis for such a response.
We think this is a huge validation of Enumeral’s platform. Although terms of the agreement were not disclosed, Merck will provide R&D funding to Enumeral. Additionally, Enumeral is eligible to receive undisclosed future milestone payments if certain goals are achieved. Merck has exclusive rights to data related to its proprietary compounds that are generated from the studies.
An Introduction To Enumeral Biomedical
Enumeral’s mission is to discover and develop best-in-class antibody immunotherapies using the company’s proprietary platform that uniquely leverages human cell biology.
Enumeral’s microengraving technology integrates proprietary and standard laboratory processes, along with data acquisition and software, to link multiple parameters of cell function with high throughput and sensitivity target screening. The platform uses a proprietary chip which contains on its surface a soft molded dense array of spatially addressable subnanoliter microwells affixed to a glass slide, and standard laboratory equipment such as manual pipetting devices, slide washers, microscopes and microarray readers. The device is currently manufactured from polydimethylsiloxane (PDMS).
The first component of the platform process is a thin PDMS chip of the size of a standard microscope slide. It may be configured for arrays containing ~85k or ~250k 0.05 mm microwells, depending on the experimental protocol. These microwells are 1/60th the size of conventional 3.0 mm wells, allowing for substantially greater sample sensitivity.
The second component of the process is a glass microscope slide that has been functionalized (i.e. coated with capture reagents) to enable it to create a “printed” microarray representation of secreted proteins from individual cells. Each microwell is then attachment to the top of the PDMS chip, in effect sealing each individual subnanoliter microwell for a fixed period of time. Due to device configuration, each location on the now printed microarray slide is spatially registered to a corresponding microwell in the device.
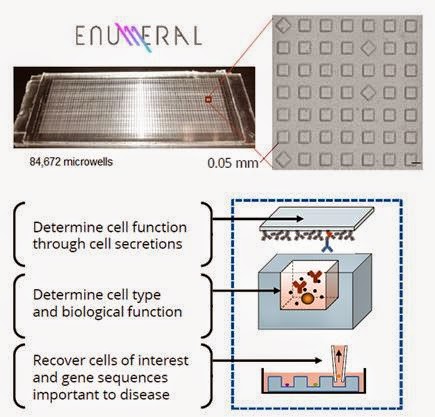
Human cells (from blood, serum, or tissue) are introduced to the chip through simple non-specified pipetting (no pre-processing required) and allowed to settle naturally from suspension into microwells. The cells weakly adhere to the bottom of the individual wells, essentially creating an immune system on a chip. The design of the system facilitates cell dispersion across its surface, resulting in rapid loading and analysis for preclinical validation.
Enumeral’s technology enables extensive interrogation of the immune microenvironment in human biopsy samples. The extremely small scale ensures that adequate quantities of secreted materials are available for analysis after very short incubation periods (10–60 minutes). Detectable secreted materials include antibodies, cytokines, and other pharmacologically relevant substances including cell culture. Cell types include T-Cells, B-Cells, peripheral blood mononuclear cells, mucosal, cerebrospinal fluid, and bone marrow tissue sourced from humans and mic. The platform:
– Uniquely enables the enumeration and rapid identification of rare immune cells critical to immune responses;
– Facilitates the analysis of function and the lineage of the cells in an unbiased fashion;
– Allows the analysis of cell surface markers and numerous types of cell secretion (antibodies, cytokines) through its proprietary micro-engraving process;
– Enables the recovery of individual cells – while they are still alive – in order to obtain single cell gene sequence information (linking function and genetics) through reverse transcription polymerase chain reaction (RT-PCR).
The entire process is designed to allow pre-clinical validation of targets by using human tissue samples, as oppose to conventional animal (murine) or in vitro approaches to discovery and target validation. However, research and animal models are generally not predictive of human response. Enumeral believes that the more human information that can be leverage prior to clinical studies, the higher the success rate will be and the more likely it is that those selections will be “best-in-class” candidates. We call this “rational target validation”.
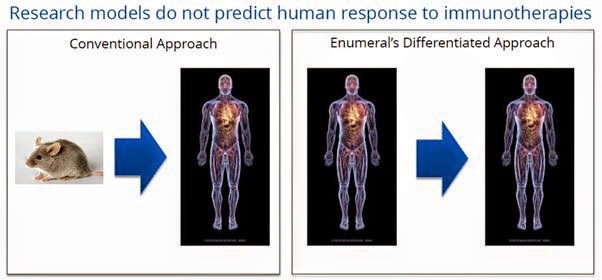 The Platform
The Platform
Therapeutic Discovery: Inputs are cellular libraries that are derived from the company’s own target-specific mouse immunization campaigns or cellular libraries from human patient donors. Antibody hits and leads are the output. These hits and leads are further characterized for biological activity, confirmation of which may lead to drug candidates that can be brought to clinical trials. It is important to note that Enumeral’s platform enables rapid, low-cost generation of proprietary libraries, removing the need for in-licensing potentially expensive third-party libraries.
Immune Profiling: Inputs are tissue samples sourced from patients or commercial vendors, and the outputs are single cell functional profiles including assessment of the frequencies of functional immune cells. When such assays are carried out with cells in the presence of potential antibody candidates, as a way of measuring the potential modulation effects of the tested candidate, such information will enable the company to rationally select antibody therapeutic candidates with the best immune cell targeting properties – that is, those candidate that are observed, through application of the platform, to interact with and activate or modulate appropriate effector cells preferentially over potentially harmful inflammatory or other damaging cells.
We believe Enumeral’s antibody discovery technology holds multiple advantages over currently available technologies. For example, Enumeral’s technology has the capability to analyze primary patient-derived biopsy tissues to mine the human immune system for information that is difficult to obtain using conventional methods. Data obtained may guide the development of new and more effective therapeutic and diagnostic products. The technology is designed to increase the efficiency, sensitivity, and probability of finding rare cell types associated with disease or drug response, as well as rare and previously unknown antibodies that may have the potential to become novel therapeutic candidates.

Enumeral believes its technology platform allows for faster and more efficacy target discovery and lead optimization. One way the technology accomplishes this goal is by performing drug candidate validation at the single cell level. This analysis can determine, even on very few immune cells, whether or not the relevant target proteins are expressed and the variety of cell types upon which they are displayed. Enumeral scientists can then use this information to determine things like beneficial effect, toxic effect, or a disease state. These single cell functional profiling capabilities provide for a deeper understanding of the variety of human responses, and thus, may provide a rational basis to guide product design and development.
Enumeral’s technology platform can also improve the prospects of antibody discovery programs by enabling quantitative screening of antibody-secreting cells in a population of cells without introducing bias from prior sorting according to cell surface markers or other low-yield transformation and handling steps. The platform is also agnostic as to the species of cell sample origin and works on human, mouse or rabbit models.
When applied to human cells, Enumeral’s technology increases the probability of finding rare antibodies that have the characteristics essential to becoming safe and effective drugs while also providing for an assessment of antibody affinity, specificity and potential tolerability much earlier in the discovery and development process than with conventional techniques. Further, quantifying individual human immune response directly in blood or biopsy samples should enable the development of immune-based diagnostic products for the classification of responders and non-responders to therapeutics.
With Enumeral’s platform, individual cell secretions can be analyzed while retaining viable cells for further analysis. Other assay methods for determining cell function and phenotype (ELISpot, FACs, et. al.) that are common to other approaches usually result in the death of the cells under study. In addition, further sequential rounds of micro-engraving onto additional multiplexed capture slides can be performed, greatly expanding the analytic capability of the platform.
After cells that generate antibodies of interest are identified, the individual cells are retrieved and RT-PCR is performed in order to obtain DNA sequence information corresponding to the potential antibodies. Enumeral’s single cell RT-PCR recovery rates for matched heavy and light DNA sequences are typically greater than 50%, with 80-90% for antibody heavy chain and light chain genes individually, compared to the industry standard of around 50% for heavy chains, and significantly less for antibody light chain genes.
Internal Programs
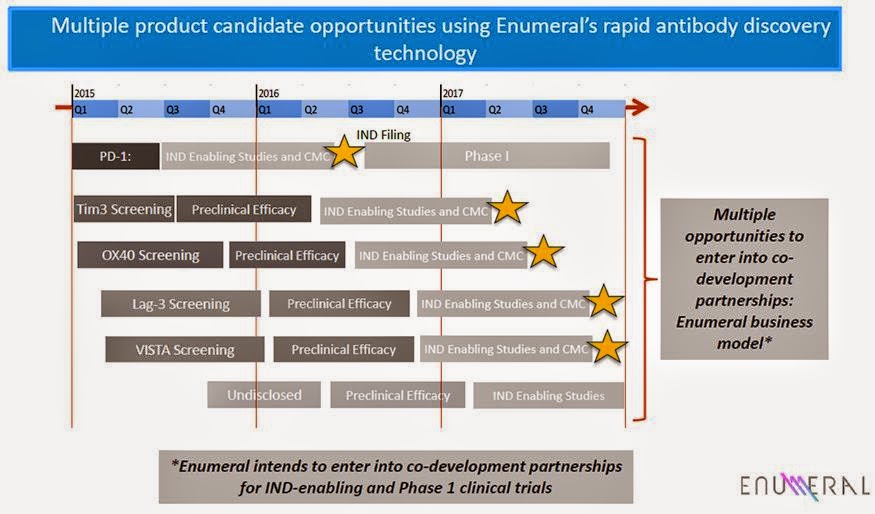
Enumeral is currently developing antibodies against three checkpoint protein targets for the treatment of cancer and other diseases. All three targets, PD-1, OX40, and Lag-3, along with CTLA-4, are a group of functionally-related proteins expressed on various immune cells, including T cells, that together function as immune checkpoints. Management plans to generate superior antibodies for these three targets for both in-house development and co-development with partners. Target therapeutic areas include cancer, inflammatory diseases, autoimmune diseases, and infectious diseases. Management plans to use its proprietary platform technology to determine rational development strategies across these disease areas.
For example, Enumeral has developed assays to identify animal- and human-derived native antibodies that bind to PD-1, OX40, and Lag-3. Initial screens have identified hundreds of antibody candidates that bind to PD-1, with a subset undergoing initial preclinical validation. Characterization of additional antibodies is ongoing, and proprietary libraries directed at OX40 and Lag-3 are being screened. Management’s goal is to initiate partnering discussions for the company’s PD-1 program here in 2015.
In order to accelerate the development of antibody therapeutics, Enumeral is also developing assays to stratify patients with various cancers and other diseases that might benefit from immunomodulatory treatment. Disease-specific assays can be rapidly developed for each type of patient population using the company’s technology. Such assays will be applied in ex vivo “human preclinical” trials using biopsies from the intent-to-treat population to select lead candidates that exhibit the best immunomodulatory profiles, including appropriate activation of immune cells that target the tumor cells. The company will also apply the assays for measurement of the potential for more general unwanted immune-related effects, such as inappropriate cytokine release or lymphocyte activation, especially immune-suppressive or tumor-promoting lymphocytes, to aid in selection of best-in-class lead candidates.
The development of stratification tools will enable predictive decision making during development, which should lead to faster, less costly, and ultimately more successful clinical development.
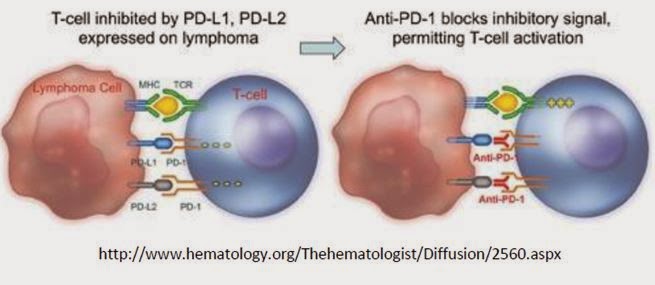
 …Anti-PD-1…
…Anti-PD-1…
Enumeral started the screening for anti-PD-1 antibodies from immunized rodents at the end of the fourth quarter of 2013. This work led to the identification of novel antibodies that bind to PD-1 in vitro and expressed on the surface of T cells. Multiple diverse antibody sequences were identified in the screening, two of which contain similar sequences to two different Phase 3 product candidates, Merck’s pembrolizumab and Bristol-Myers’ nivolumab, indicating the potential of Enumeral’s platform to rapidly identify commercially-relevant antibody candidates. Two lead antibodies have been demonstrated to be functionally similar to others described in the literature. Ongoing preclinical evaluation is aimed at determining which candidates demonstrate optimal ‘ex vivo’ human functional characteristics.
Following preclinical evaluation and lead nomination, Enumeral plans to identify partners and contract research organizations (CROs) to undertake the required preclinical disease model and IND-enabling safety and toxicity studies, as well as formulation and CMC package development for the IND filing. Management currently expects to have such preclinical and ex vivo human data packages on its first program during the first half of 2015, and the second and third programs, within the later in the year. If timelines hold, we estimate the first Phase 1 clinical trial could start in 2016.
 Collaborative Partnerships
Collaborative Partnerships
As an early development stage biotech company, Enumeral seeks to enter into collaborative partnerships for product discovery, development, and commercialization. We believe these partnerships are important to Enumeral in the following aspects:
– Partnership can accelerate the development of product candidates through the potential leverage provided by large companies, including, research and development capabilities, manufacturing, clinical and regulatory expertise, and sales and marketing.
– Partnership further validates the company’s technology and the potential of its candidates;
– Partnership can boost the company’s balance sheet by providing non-dilutive financing in terms of upfront, milestones, and royalty payments.
Enumeral has completed several collaborations with four leading pharmaceutical companies in the form of proof-of-concept projects that have provided for modest cash payments (in the hundreds of thousands of dollars per project). The most recent agreement with Merck was signed in December 2014. We anticipate management entering into one or more additional large-scale corporate collaborations in the next twelve months.
We believe that such large-scale collaborations provide platform validation and meaningful revenues that will reduce future operating losses and equity financing requirements through a combination of up-front payments, subsequent milestone payments as the candidate clears pre-clinical and clinical regulatory hurdles, and royalty payments on future sales of the marketed drug.
Financial Update
On March 19, 2015, Enumeral Biomedical Holdings, Inc. (ENUM) reported financial results for the fourth quarter and full year ending December 31, 2014. Total revenues in the quarter were $0.04 million, down sizably from the fourth quarter 2013 on the conclusion of certain collaboration contracts and grants, but still generally in-line with our financial modeling. For the full year 2014, revenues totaled $0.16 million. Operating loss in the fourth quarter totaled $2.01 million, driven by $1.04 million in R&D and $1.01 million in G&A. For the full year 2014, R&D expense was $3.58 million and G&A was $3.02 million, leading to an operating loss of $6.43 million. Net loss in the full year totaled $8.18 million and included changes in the fair-value of outstanding warrant liabilities, which is a non-cash charge. Actual operating cash burn in 2014 was only $6.33 million. Loss per share for the full year was $0.26.
Enumeral exited 2014 with $13.47 million in cash and investments on the books. As noted above, the company burned $6.33 million in cash from operating activities. We expect a slight ramp in both R&D and G&A costs in 2015, but believe the current cash balance is sufficient to fund operations for at least the next 12+ months. We remind investors that in August 2014, Enumeral completed a $21.5 million private placement financing. The company sold 21.5 million units at a price of $1.00 per unit. Each unit includes one share of common stock and a warrant to purchase one share of common stock at an exercise price of $2.00 per share for a five-year period.
Conclusion
In addition to its internal programs, Enumeral also plans to monetize its technology by establishing partnerships with big pharma or biotech companies, the most recent agreement with Merck was signed in December 2014. Prior to the Merck collaboration, the company already had multiple proof-of-concept partnerships with a few big pharma companies. We anticipate more deals coming in 2015. We believe such large-scale collaborations will not only provide non-dilutive financing to the company but also further validate Enumeral’s technology and programs.
We acknowledge that Enumeral is still in the early development stage with a Phase 1 trial starting in 2016. But when we look at the company in detail and the industry in which it operates, we realize that this company has the potential to grow dramatically in the next few years. The unique platform technology not only provides the basis for rapidly building a robust pipeline, but also forms the basis for corporate partnerships that will generate near-term revenue for the company.


