Last month I wrote a detailed article introducing investors to 22nd Century Group, Inc. (NYSEMKT:XXII), a Clarence, NY-based biopharmaceutical company founded in 1998 with proprietary technology in nicotine biosynthesis and tobacco plant-biotechnology. The company is engaged in the development and commercialization of tobacco harm reduction cigarettes and smoking cessation products, as well as the contract manufacturing of premium and super-premium brand cigarettes out of the company’s 61,000 square foot MSA-authorized manufacturing facility in Mocksville, NC.
My introductory article provides a good summary of the company’s research and development activities, which includes an exclusive worldwide licensing agreement with British American Tobacco (NYSE:BTI), one of the world’s largest producers and distributors of tobacco products. I also discuss in my introductory article the company’s two commercial products in Red Sun®, a super-premium brand cigarette that contains roughly twice the amount of nicotine versus leading premium brand cigarettes and MAGIC®, a very low nicotine (VLN) cigarette that contains roughly 95% less nicotine per cigarette versus commercially available brands. For the purpose of this article, I will highlight the company’s efforts in gaining approval for modified risk cigarettes, Brand-A, and with X-22, the company’s Phase 3-ready prescription cigarette for smoking cessation.
Update On Modified Risk Tobacco Product
22nd Century’s goal is to be the first company to gain approval for a Modified Risk Tobacco Product (MRTP) in 2016. As a quick reminder, almost all tobacco marketing practices ceased in the U.S. following the tobacco Master Settlement Agreement (MSA) of 1998. The MSA settled Medicare lawsuits targeting major tobacco manufacturers, banned almost all commercial advertising of tobacco products, seeded anti-smoking advocacy groups, and funded education and awareness for smoking-related diseases. In 2009, the U.S. Congress passed The Family Smoking Prevention and Tobacco Control Act (TCA), which gave the U.S. FDA broad authority to regulate the manufacture, distribution, and marketing of tobacco products in the U.S. That is when additional restrictions on advertising and promotion came into play for the industry, and the U.S. FDA took over review of “modified risk” language on product labels to prevent potentially misleading claims.
Since the Tobacco Control Act of 2009, tobacco cigarette products can no longer use terms like “light,” “ultra-light,” “low tar,” or “all-natural.” To gain approval for these types of claims, tobacco manufacturers must file applications with the U.S. FDA for review. The application must contain clinical data supporting the modified risk claim.
On December 31, 2015, 22nd Century Group submitted a MRTP application to the U.S. FDA for Brand-A, a code name for the company’s modified risk VLN cigarette that contains roughly 95% less nicotine per cigarette versus commercially available brands. If approved, Brand-A is expected to be the first cigarette with a modified risk claim in the U.S. The company already manufactures MAGIC®, a commercially available and separate VLN cigarette, without the modified risk claim. Sales of MAGIC® are slowly ramping in Europe; however, it is safe to say that Brand-A, with a modified risk claim, will see significant acceleration in sales over the current trajectory with MAGIC® based on consumers desire to either quit smoking, smoke fewer cigarettes, or reduce the harm associated with smoking tobacco products.
For example, according to research done by JP Morgan, 90% of smokers would be willing to try a new brand if it were “safer” than their usual brand. This is evident by the fact that “light” cigarettes had 83.5% of the market prior to the TCA in 2009 that banned the use of that term. Roughly 45 million American’s smoke and retail sales of cigarettes in the U.S. were approximately $75 billion in 2014. A product that appeals to 90% of the market is potentially a very big product!
U.S. Government Support
The concept of a VLN cigarette like Brand-A or MAGIC® as a means to help individuals quit smoking or reduce the harm from smoking has gained significant steam over the past several years. The idea has even has peaked the interest of some of the country’s leading public sector institutes, including the National Institute on Drug Abuse (NIDA), the U.S. Food and Drug Administration (FDA), the U.S. National Cancer Institute (NCI), and the U.S. Centers for Disease Control and Prevention (CDC).
In August 2010, affiliates from the above organizations, RTI International, and 22nd Century Group got together to design “research cigarettes” with varying levels of nicotine, to be sold in the U.S. market so that the U.S. government can better understand smoking habits and addiction. 22nd Century Group was selected to participate in this study due to the company’s unique proprietary technology and ability to grow tobacco across a wide spectrum of nicotine levels.
Since the initial sub-contract, approximately 22 million SPECTRUM® brand cigarettes, in 24 styles with eight varying levels of nicotine, have been ordered to date, with the most recent 5.0 million purchase order from NIDA coming in September 2015. This is expected to generate approximately $0.6 million in revenues to 22nd Century Group.
22nd Century Group has since expanded its supply and distribution of SPECTRUM® brand cigarettes and is also looking to sign up additional research agreements with accredited organizations interested in purchasing tobacco cigarettes with varying levels of nicotine in the future. The monopoly that 22nd Century has on the proprietary technology to significantly alter the nicotine content in tobacco means that 22nd Century is the sole source of such unique products.
The Clinical Data Supports Use Of VLN Cigarettes To Reduce Harm
In October 2015, results from a randomized, double-blind, parallel-design trial using VLN SPECTRUM® brand cigarettes were published in The New England Journal of Medicine (NEJM). The 840 participant, six-week study (NCT01681875) took place between June 2013 and July 2014 at ten sites around the U.S. Eligibility criteria included adults smoking of five or more cigarettes per day with no current interest in quitting smoking. Participants were randomly assigned to smoke for six weeks either their usual brand of cigarettes or one of six types of investigational SPECTRUM® cigarettes provided for free. The investigational cigarettes had nicotine content ranging from 15.8 mg per gram of tobacco (typical of commercial brands) to 0.4 mg per gram (VLN). The primary outcome was the number of cigarettes smoked per day during week six.
Results were quite astonishing. The first thing investigators learned was that smoking VLN cigarettes resulted in less total nicotine equivalents per nmol/mg of creatinine in excreted urine (see below).
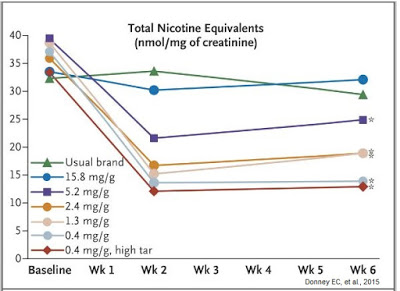
Ok, this may not be a ground-breaking discovery because it seems obvious that smoking cigarettes with less nicotine result in lower exposure to nicotine, but the graph above clearly demonstrates that smokers were not increasing the number of cigarettes they smoked per day to try to maintain a baseline nicotine level. Additionally, the data show individuals smoking the VLN type cigarettes had no increase in expired carbon monoxide level or total puff volume (i.e. they were not inhaling more smoke), suggesting minimal compensation increase when exposed to VLN cigarettes.
In fact, the opposite happened. Not only did subjects smoking VLN cigarettes have lower nicotine levels compared with control cigarettes, they also reported reduced dependence on nicotine, as well as reducing cravings during abstinence from smoking. This can be seen in the two graphs below.
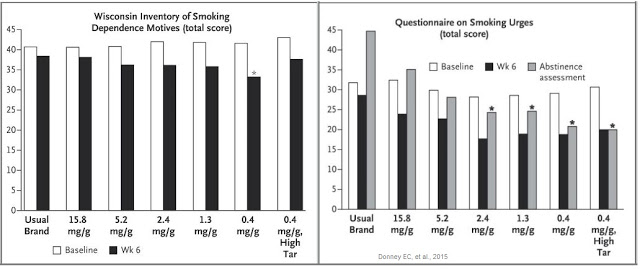
The combination of reduced nicotine exposure, reduced dependence, and reduce urges to smoke led to fewer cigarettes smoked per day for individuals randomized to VLN cigarettes (14.9) than those assigned to their usual brand (22.2), and that smokers of VLN cigarettes doubled their quit attempts versus smokers of conventional cigarettes over the course of the study.
The study’s lead author, Dr. Eric Donny, explained in an article posted on USAToday.com, “The evidence is getting stronger that reducing nicotine reduces smoking and makes people less addicted to cigarettes and, in doing so, might make them more likely to quit.” Just as important to smokers is the fact that The New England Journal of Medicine articles reflected that smokers had negligible nicotine withdrawal symptoms when using the VLN cigarettes from 22nd Century.
Including the study published above in the NEJM, I have found six additional independent clinical studies that demonstrated efficacy and improved smoking cessation with 22nd Century’s proprietary VLN cigarettes. Below is a quick review of these studies and the data.
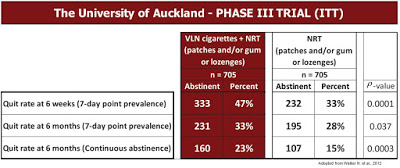
– Phase 3: Clinical Trials Research Unit, University of Auckland: This study published by Walker N, et al., 2012 was designed as a single-blind, parallel group randomized trial to determine the effects of VLN cigarettes when combined with nicotine replacement therapy (patches and/or gum or lozenges) over a six month period. A total of 1,410 participants were randomized (705 in each arm). Results show a clear increase in quit rate (33% vs. 28%, p=0.037) for the VLN+NRT group compared to NRT alone, as well as continuous abstinence (23% vs. 15%, p=0.0003) and longer time to relapse (2 months vs. 2 weeks) for the VLN+NRT group.
 – Phase 2: University of Minnesota Masonic Cancer Center Follow-up Study: This study published by Hatsukami DK, et al., 2013 randomized subjects between three groups: (1) VLN cigarettes, (2) nicotine patch, or (3) the combination VLN cigarettes + nicotine patch. Results show the combination approach led to lower rates of smoking assigned cigarettes compared to either NRT or VLN cigarettes alone, as well as less withdrawal severity when switching from usual brand to assigned product and less smoking of usual brand cigarettes during treatment.
– Phase 2: University of Minnesota Masonic Cancer Center Follow-up Study: This study published by Hatsukami DK, et al., 2013 randomized subjects between three groups: (1) VLN cigarettes, (2) nicotine patch, or (3) the combination VLN cigarettes + nicotine patch. Results show the combination approach led to lower rates of smoking assigned cigarettes compared to either NRT or VLN cigarettes alone, as well as less withdrawal severity when switching from usual brand to assigned product and less smoking of usual brand cigarettes during treatment.
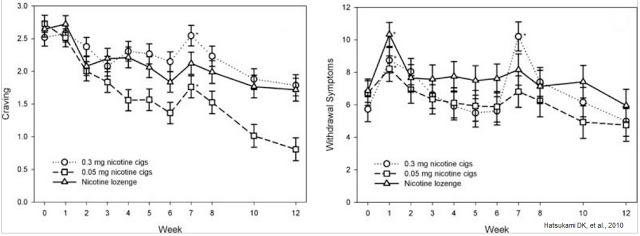
– Phase 2: University of Minnesota Masonic Cancer Center: This study, also published by Hatsukami DK, et al, 2010, was the author’s initial work with reduced nicotine cigarettes. The six-week study showed that subjects randomized to 0.05 mg cigarettes smoked fewer cigarettes per day than subjects smoking 0.30 mg cigarettes. The authors work shows that use of VLN cigarettes as a means to quit smoking might be an effective strategy because patients reported lower craving and withdrawal symptoms over the course of the study compared to higher nicotine cigarettes and nicotine lozenges (see below).
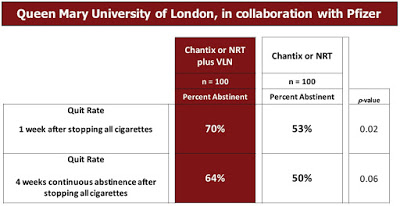
 – Phase 2: Queen Mary University / Pfizer: This study, supported by pharmaceutical giant Pfizer (NYSE:PFE) was a 200-person Phase 2 program (NCT01250301) that aimed to determine if using a behavioural replacement for smoking (VLN cigarettes), in addition to standard treatment can reduce urges to smoke over the first four weeks of abstinence. All subjects received standard smoking cessation treatment, which was a combination of stop smoking medication (e.g. nicotine replacement therapy and varenicline) and motivational support, with half also receiving VLN cigarettes. Results showed that subjects smoking VLN cigarettes in addition to standard therapy maintained abstinence at a greater rate than standard therapy alone (see below).
– Phase 2: Queen Mary University / Pfizer: This study, supported by pharmaceutical giant Pfizer (NYSE:PFE) was a 200-person Phase 2 program (NCT01250301) that aimed to determine if using a behavioural replacement for smoking (VLN cigarettes), in addition to standard treatment can reduce urges to smoke over the first four weeks of abstinence. All subjects received standard smoking cessation treatment, which was a combination of stop smoking medication (e.g. nicotine replacement therapy and varenicline) and motivational support, with half also receiving VLN cigarettes. Results showed that subjects smoking VLN cigarettes in addition to standard therapy maintained abstinence at a greater rate than standard therapy alone (see below).
 – Phase 2: Vector Tobacco: This was a randomized double-blind, active controlled, parallel group, multi-center Phase 2 clinical trial conducted to evaluate the efficacy of reduced-nicotine cigarettes as a novel smoking cessation treatment. Treatment consisted of Quest brand of cigarettes (Quest 1, 2, and 3), which respectively deliver 0.59+/-0.06, 0.3+/-0.05, and less than 0.05 mg nicotine, either alone or in combination with nicotine replacement therapy (NRT). The primary endpoint was four weeks of continuous abstinence (Weeks 7-10), with additional follow-up at 3 and 6 months. A total of 346 subjects were treated. Results were published by Becker KM, et al., 2008 and showed that Quest plus NRT was more effective than active control plus NRT in achieving four weeks of continuous abstinence (32.8% vs. 21.9%). No serious adverse events were attributable to the investigational product.
– Phase 2: Vector Tobacco: This was a randomized double-blind, active controlled, parallel group, multi-center Phase 2 clinical trial conducted to evaluate the efficacy of reduced-nicotine cigarettes as a novel smoking cessation treatment. Treatment consisted of Quest brand of cigarettes (Quest 1, 2, and 3), which respectively deliver 0.59+/-0.06, 0.3+/-0.05, and less than 0.05 mg nicotine, either alone or in combination with nicotine replacement therapy (NRT). The primary endpoint was four weeks of continuous abstinence (Weeks 7-10), with additional follow-up at 3 and 6 months. A total of 346 subjects were treated. Results were published by Becker KM, et al., 2008 and showed that Quest plus NRT was more effective than active control plus NRT in achieving four weeks of continuous abstinence (32.8% vs. 21.9%). No serious adverse events were attributable to the investigational product.
– Phase 2: Roswell Park Cancer Institute: This Phase 2 study, published by Rezaishiraz H, et al., 2007, investigated whether treatment with the combination of denicotinized cigarettes and 21-mg nicotine patch for two weeks before a designated quit date could lessen cravings for smoking, thereby helping smokers abstain from smoking. Half of the subjects received two weeks of a combination of denicotinized cigarettes and 21-mg nicotine patch for two weeks before the quit date; the remaining smokers were switched to light cigarettes during the two weeks before the quit date. After the quit date, all subjects received counseling for smoking cessation and were provided nicotine patches for up to eight weeks. Self-reported cravings for smoking, withdrawal symptoms, and smoking abstinence were measured at predetermined intervals using phone-based surveys and in clinical visits.
The group that used denicotinized cigarettes and nicotine patch before quitting reported less frequent and less intense cravings for cigarettes in the two weeks before and after the designated quit date. Self-reported withdrawal symptoms and quit rates did not differ significantly between the groups. The authors concluded that the use of a denicotinized cigarette combined with the nicotine patch appears to lessen cravings to smoke in the immediate post-cessation period.
X-22 – The Prescription Cigarette
Beyond the commercial strategy with MAGIC® and Brand-A, 22nd Century Group has a Phase 3-ready asset in X-22, designed to be the world’s first prescription-brand cigarette. The independent clinical data generated to date with VLN cigarettes like SPECTRUM® and Quest suggests that the concept of a VLN cigarette is helpful in reducing cravings and lowering withdrawal symptoms for cigarettes, which can result in fewer cigarettes smoked per day and a greater chance of quitting.
Unfortunately for 22nd Century Group, clinical data with X-22 has been less encouraging than the seven studies outlined above. Back in December 2011, the company reported results from a Phase 2b study with mixed results. The data showed a reduction of smoking from baseline over the six-week treatment period, as well as an 89% reduction in the median number of cigarettes smoked compared to the baseline, but the results were not statistically significant when compared to the active control, a cigarette containing conventional nicotine levels.
Results from the Phase 2b study have not been published, so I have not been able to fully delve into why this trial failed. The results are quite surprising given all the other studies that support the concept of VLN cigarettes. Management believes that X-22 may have gone slightly too far in reducing the nicotine content of X-22. The company has since reformulated X-22 to include about twice the nicotine level used in the Phase 2b study.
I do not anticipate management at 22nd Century Group moving X-22 into Phase 3 trials without external support. The most recent SEC filing notes that the company has, “Identified and met with several potential strategic partners towards funding Phase 3 clinical trials for our X-22 prescription smoking-cessation aid in development.” I believe the Phase 3 program will likely cost in the area of $20-30 million to complete, so this is not a substantial investment by a strategic partner, assuming management is talking to the industries biggest players.
It would make sense to talk to the biggest players about X-22 because the smoking cessation market is enormous. According to the U.S. CDC, approximately 50% of U.S. smokers attempt to quit smoking each year, but only 2% to 5% actually quit smoking in a given year. It takes smokers an average of 8 to 11 “quit attempts” before achieving long-term success. Approximately 95% of “self-quitters” (i.e., those who attempt to quit smoking without any treatment) relapse and resume smoking.
Smokers currently have limited choices of FDA-approved products to help them quit smoking. These include Pfizer’s Chantix® (varenicline) and Glaxo’s Zyban® (bupropion). Chantix® was introduced in the U.S. market in the fourth quarter 2006. Since 2007, Chantix® has been the best-selling smoking cessation aid in the U.S., with sales, according to Pfizer Inc., of approximately $701 million in 2007. Unfortunately for Pfizer, the U.S. FDA slapped a “black box” warning on the drug for serious neuropsychiatric events, including suicidal ideation, and increased risk of harmful cardiovascular events. Chantix® sales have been flat ever since. Zyban® has a similar black box warning.
Non-prescription options are dominated by nicotine replacement therapy (NRT) products, which includes gums, lozenges, patches, nasal sprays, and inhalers. These products offer only minimal efficacy and do nothing to address the behavioral or psychological addiction to smoking. E-cigarettes, all-the-rave for many people, offer mixed results of actually helping people quit and may even have serious health concerns, such as cancer. Despite these issues, the smoking cessation market is currently $5.0 billion in the U.S. and expected to nearly triple in the next few years.
I’m not saying X-22 is the end-all-be-all of smoking cessation products; the failure of the Phase 2b trial certainly creates doubt in the mind of investors. That being said, this is a potential billion-dollar product. As such, 22nd Century Group is actively looking for a partner and with minimal necessary funding, it is possible to see the Phase 3 trial start at some point in 2016. I think this could act as a major catalyst for the shares should a deal get done.
Conclusion
22nd Century Group is positioned for significant growth in 2016. The commercial business with Red Sun® and MAGIC® is finally starting to take off. In the U.S., Red Sun® is being distributed at premium tobacco outlets with more than 800 locations around the U.S. and MAGIC® is slowly building momentum in Europe. Financial results for the three previous quarters have exceeded Street expectations, and forecasts for 2016 have doubled over the past year (source: Chardan Capital).
The potential MRTP approval of Brand-A, the first in the U.S., could have a tremendous impact on the company’s business. Being able to claim that Brand-A is the first and only “reduced harm” cigarette on the market will surely lead to market share gains for the product. Vector Tobacco Inc. was able to grow its Quest 3 product, a VLN cigarette similar to MAGIC® to $60 million in wholesales sales, or about 26 million packs in 2007. End-user sales were likely in the area of $75 million. This is the commercial potential for MAGIC® in the U.S. I think Brand-A, which I view as MAGIC® with the MRTP language, as having ten-fold upside.
As such, I think the industries biggest players, Philip Morris, Reynolds America, British American Tobacco, Japan Tobacco, and Imperial Tobacco, no doubt will be watching for approval of the MTRP application later in 2016. The initial success with SPECTRUM® and previous success of the Quest 3 product clearly validates the market, and the strong clinical data backing up claims of reduced smoking and improved odds of quitting once the MTRP application is approved should resonate with 90% of the market.
The wildcard for 2016 is X-22. Data from the NIDA sponsored SPECTRUM® trial recently published in The New England Journal of Medicine clearly supports the idea of a VLN cigarette as a means to reduce harm and improve the odds of cessation. Independent clinical studies and work by competitors (e.g. Pfizer, Vector Tobacco) further validates the concept of X-22. It will be interesting to see if management can close a deal in 2016 to move X-22 into Phase 3.


